
Understanding the behavior of bubbles on the electrode surface and in the electrolyte is crucial to the design and optimization of the electrochemical process.
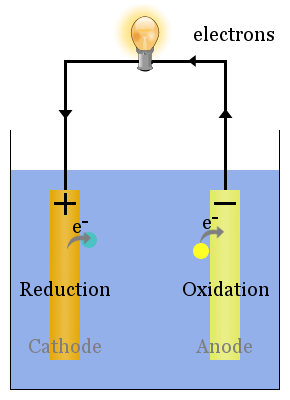
Many electrochemical processes involve gas evolution and bubble generation on the electrodes. The results indicate that the cyclic voltammetry response of the whole porous sulfur cathode could be solved as a response of a single particle, which might lead to a simplification of numerical simulations. The model was used to model cyclic voltammetry at a planar electrode and at single porous particles. The redox mechanism was described through a multi-step mechanism, with kinetics being defined through the Butler-Volmer equation. The model describes a multi-component transport of species through Nernst-Plank equations in a dilute solution. This paper presents a custom electrochemical model of a lithium-sulfur cell implemented into Ansys Fluent. Numerical simulations of the processes occurring in lithium-sulfur batteries might bring a better understanding of the governing mechanisms, which will allow the necessary development of the applicable cells. However, their performance is hindered by poor cyclability, self-discharge and capacity loss. The lithium-sulfur batteries have the potential to become a leading energy storage system as they are showing promising characteristics such as high theoretical capacity and energy density. The model has predictive power and, after simple calibration, can be used to predict the performance of REM anodic oxidation systems. Because the presence of bubbles increases the hydraulic resistance, the residence time of paracetamol-and consequently its degradation degree-increases, but the productivity goes down. This behavior is due to a decrease in the generation of oxygen during the recombination reaction of the hydroxyl radicals, which are consumed in the oxidation reaction of the organic compounds. It was shown that in the solution that does not contain organic components, gas bubbles form faster and occupy a larger pore fraction than in the case of the presence of paracetamol with an increase in the paracetamol concentration, the gas fraction decreases. It was found that the absolute value of the zeta potential of the pore surface decreases with time, which leads to a decrease in the permeate flux due to a reduction in the electroosmotic flow. The processing of the experimental data was carried out. We propose a simple one-dimensional non-stationary model of the transport of diluted species during the anodic oxidation of paracetamol using REM to describe the above effects. This work aims to study the processes underlying the reduction in the efficiency of anodic oxidation, and in particular the formation of gas bubbles and the recharge of the REM pore surface at a current density exceeding the limiting kinetic value. The main problem with REM is the formation of fouling and gas bubbles in the pores, which leads to a decrease in the efficiency of the process because the hydraulic resistance increases and the electrochemically active surface is shielded. The use of reactive electrochemical membranes (REM) in flow-through mode during the anodic oxidation of organic compounds makes it possible to overcome the limitations of plate anodes: in the case of REM, the area of the electrochemically active surface is several orders of magnitude larger, and the delivery of organic compounds to the reaction zone is controlled by convective flow rather than diffusion. The work points out the feasibility and problems of zinc electrowinning under high current density situation, which could provide necessary reference the electrowinning field. The increase of current density on the electrolytic cell and electrode plate is the direct cause of accelerating zinc deposition and aggravating anodic corrosion. Finally, the distribution of secondary current density in the electrolytic cell was simulated by the method of computational fluid dynamics. In addition, the corrosion behavior of Pb-Ag anode at high current density was studied, which suggest that Pb-Ag anode displays higher surface roughness and lower oxygen evolution overpotential at high current density with the main corrosion phase of β-PbO2. The XRD analysis of the zinc plates at different current densities revealed that high current densities change the zinc growth preferential orientation, leading to poor surface quality. The percentage of hydrogen evolution at current density 800 A/m² increased by 3.3% compared to 500 A/m², and the current efficiency decreased by 3.06%. The results suggest that the increase of current density increases the proportion of hydrogen evolution at the cathode and thus decreases the current efficiency.

In this work, the effects of high current density (500 A/m², 600 A/m², 700 A/m², 800 A/m²) on zinc electrodeposition as well as the anodic corrosion behavior of lead silver alloy were investigated.


 0 kommentar(er)
0 kommentar(er)
